How Cubein’s End-to-End Product Development Scaled Galanto’s Neuro-Rehabilitation Vision

Executive Summary
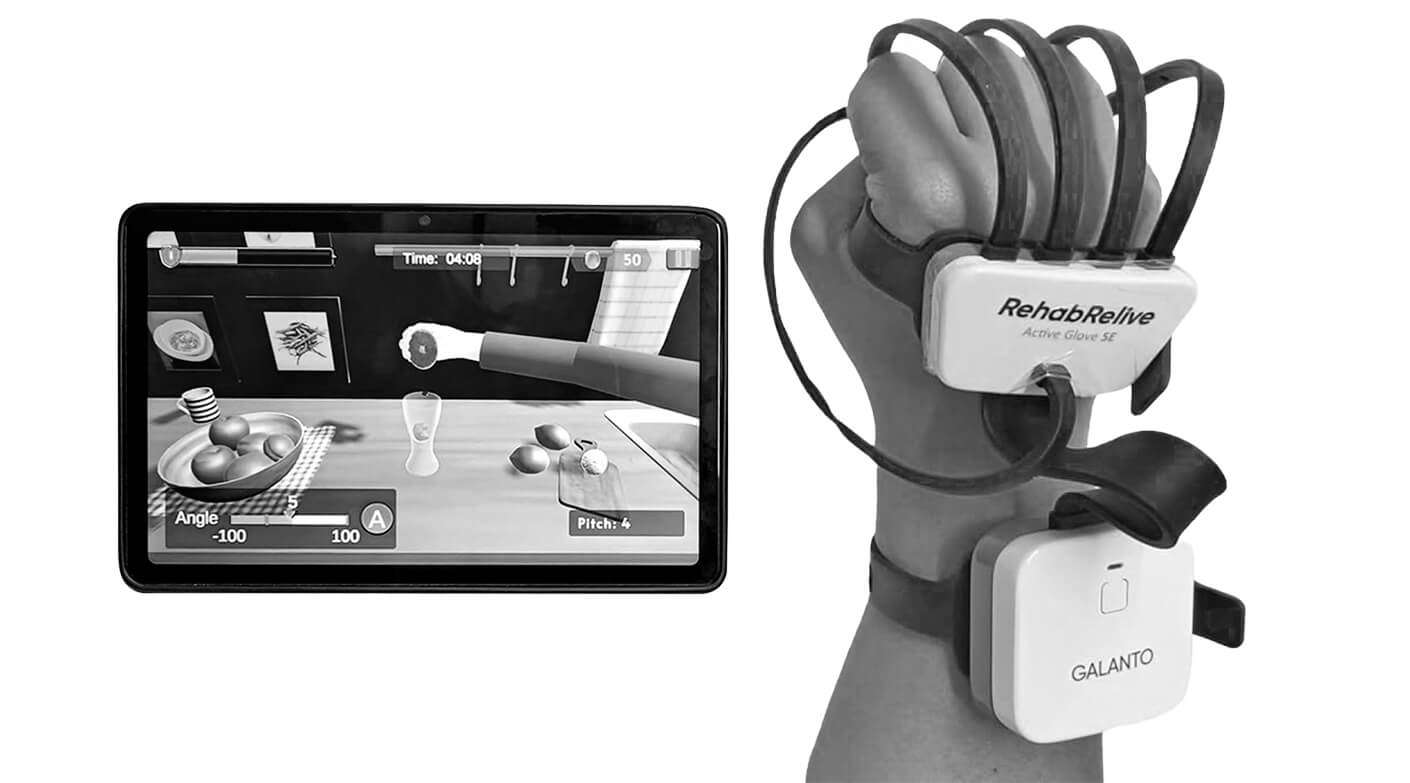
Galanto Innovations, an IIT Gandhinagar-based startup, set out to revolutionise neuro-rehabilitation in India with its flagship product, the RehabRelive Active Glove. Facing a critical 90-day deadline to launch a production-ready prototype for a major industry event, they needed a partner capable of managing the entire development process—from concept to functional units—with agility and precision. By leveraging Cubein’s end-to-end service, Galanto successfully navigated design exploration, functional prototyping, and short-run production, delivering 50 fully tested units on time. This collaboration not only accelerated their path to market but also validated a scalable, user-centric design ready for real-world impact.
The Innovator: Galanto Innovations
Galanto Innovations is on a mission to restore movement and independence to individuals suffering from neurological and orthopaedic conditions, including cerebral palsy, stroke, and traumatic injuries. Their approach combines deep clinical insight with cutting-edge technology to create accessible, effective, and next-generation rehabilitation solutions. The RehabRelive Active Glove represents a significant leap forward as India’s first comprehensive fine motor rehabilitation system.
The Challenge: Engineering a Personalised Path to Recovery
Galanto’s vision was ambitious: a wearable rehabilitation device that was not only clinically effective but also affordable, scalable, and adaptable to each user’s unique recovery journey. The device needed to outperform conventional therapy methods, be ergonomic for extended wear, and provide quantifiable progress data.
The primary challenge was the intensely compressed timeline. To capitalise on a pivotal industry meetup, Galanto required a fully functional, production-ready prototype and 50 units for real-world testing, all within 90 days. This demanded a partner with integrated capabilities to eliminate vendor handoff delays and ensure seamless progress from concept to clinical-ready hardware.
Key Technical & Operational Requirements:
- Rapid End-to-End Development: A single partner with in-house tooling as well as mass production capacity to manage design, prototyping, testing, and manufacturing.
- Ergonomic Excellence: A lightweight, flexible design is comfortable for long-term therapeutic use.
- Material Performance: High-durability materials (ABS, speciality rubber) capable of withstanding repeated mechanical stress.
- Scalability & Cost-Effectiveness: A development pathway that directly supported future mass production without significant redesign.
- Rigorous Validation: In-house testing for mechanical integrity, wear tolerance, and long-term durability.
The Solution: Cubein’s Integrated, Phase-Driven Approach
Cubein adopted a collaborative, agile methodology, working as a seamless extension of the Galanto team.
Phase 1: Deep Discovery & Strategic Design Exploration
The process began with intensive discussions with the Galento team, including meetings with their engineers, clinical partners, and end-user patients. Cubein’s team conducted comprehensive market and material analyses to benchmark against competitors and ensure economic viability. This user-centric research directly informed the initial design strategy, focusing on minimising part count for manufacturability and maximising therapeutic output.
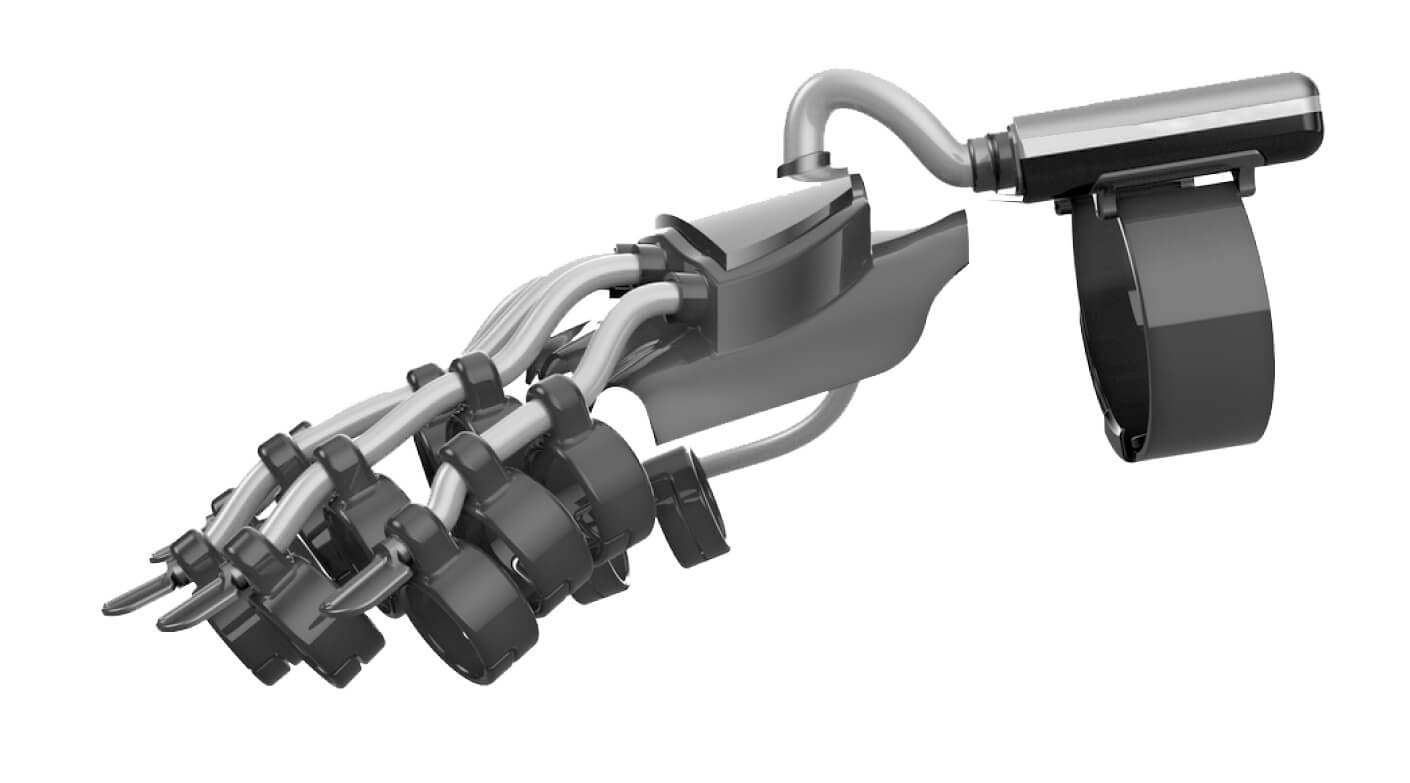
Phase 2: Iterative Prototyping for Form and Function
- FDM (Fused Deposition Modelling): 3-4 initial design variants were rapidly produced using FDM. These low-fidelity prototypes were crucial for validating core ergonomics, overall form factor, and gathering early user feedback without significant cost or time investment.
- SLA (Stereolithography): Upon design selection, high-fidelity & functional prototypes were built using SLA. These provided Galanto with parts that closely mimicked final production quality, offering superior surface finish and the necessary mechanical strength for further functional testing and demonstration.
Phase 3: Seamless Transition to Short-Run Production
With the design validated, Cubein manufactured 50 field-ready units using custom, quick-turn tooling, all executed in-house for quality control and agility. Components were produced in ABS and medical-grade rubber, finished with a glossy, easy-clean surface and precise screen printing for branding and usability.
This phase confirmed the manufacturability of the design, setting a clear pathway for future mass production using Cubein’s dedicated high-volume facilities and injection tooling expertise, once Galanto is ready to scale.
Integrated Quality Assurance:
Throughout each phase, Cubein’s in-house testing facilities were employed to rigorously validate the product. Tests included cyclic fatigue analysis to ensure longevity, tolerance checks for a perfect fit, and flexibility assessments to guarantee patient comfort. This vertical integration was critical in maintaining the aggressive timeline and uncompromising quality standards.
The Results: A Market-Ready Innovation, Accelerated
The partnership between Galanto Innovations and Cubein yielded transformative outcomes:
- Unprecedented Speed: Achieved a complete product development cycle, from concept to 50 production units, in less than 90 days.
- Capital Efficiency: Enabled a med-tech startup to launch a hardware product without the burden of massive upfront capital investment in tooling and production facilities.
- User-Validated Design: Delivered a device that met stringent ergonomic and functional requirements, directly informed by patient and therapist feedback.
- Successful Market Debut: Empowered Galanto to confidently unveil the RehabRelive Active Glove at their target industry event, generating significant interest and paving the way for future investment and adoption.
- Scalable Foundation: Established a proven, manufacturable design ready for scaling to mass production, bringing Galanto one step closer to transforming rehabilitation across India.
Cubein empowered Galanto to confidently debut the RehabRelive Active Glove, a smart, scalable, and impactful solution poised to transform rehabilitation for thousands of patients across India.
Ready to Build the Future of Med-Tech?
Partner with Cubein to bring your medical innovation from concept to clinical-ready reality, and beyond. With in-house capabilities spanning prototyping, short-run production, tooling, and mass manufacturing, we provide a seamless pathway to scale






